- HOME
- Information
- Introduction to Technologies for Continuous Manufacturing of Drug Proucts
Introduction to Technologies
for Continuous Manufacturing of Drug Products
Satisfy customers’ needs with advanced technologies
Shionogi Pharma Co., Ltd. (Shionogi Pharma, hereafter) has established a technology for continuous manufacturing of drug products. This technology is an advanced technology that will change the development and manufacturing of the drug products in the future. Continuous manufacturing has many advantages: for example, it can make scale-up study unnecessary which sometimes annoys pharmaceutical scientists during development and flexibly accommodate changing supply demands in commercial manufacturing. FDA stated in their blog*1 their idea concerning the facilitation of advanced manufacturing technologies. The blog says, “The potential public health value of advanced manufacturing is even greater in the context of ongoing COVID-19 pandemic, which has highlighted the strain on supply chain and the need for adaptive manufacturing systems to accelerate the manufacturing of medical counter measures.”
* 1 FDA Voices (August 3, 2020):
Investing in Advanced Manufacturing to Support Public Health Preparedness
A continuous manufacturing flow of a drug product is illustrated in the figure below. Shionogi Pharma can continuously manufacture a product from weighing to tableting continuously monitoring material quality at each stage.
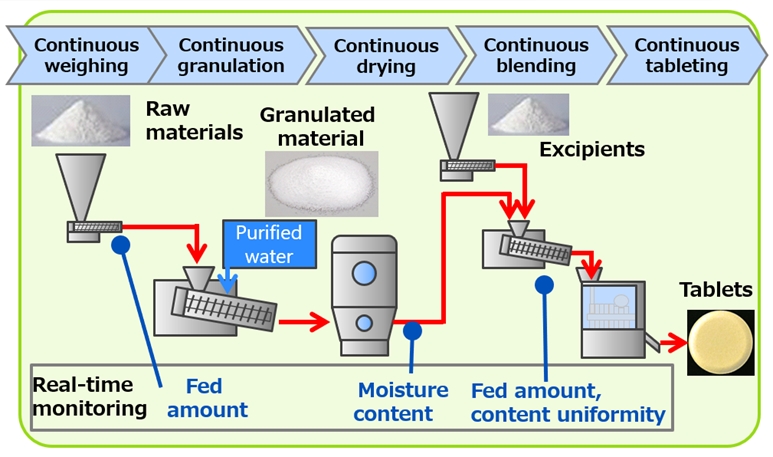
Continuous manufacturing system does not require scale-up during development. It enables us to
significantly reduce the use of valuable and expensive APIs because what you must do is only add APIs of
the amount needed to obtain drug product you require. Scale-up studies are not necessary for commercial
manufacturing. Therefore, the development period can be shortened, and study resources can be
reduced.
The amount of the drug product can be freely changed in the commercial manufacturing to accommodate
changing supply demand.
During both development stage and commercial manufacturing, processes are monitored in real time and
continuously operated. Therefore, time loss is not expected, and period of development or commercial
manufacturing are shortened. PAT (Process Analytical Technology) tools are used at each principal process
to monitor CQA (Critical Quality Attribute) or CMA (Critical Material Attribute) to ensure high quality of
the product.
In the continuous manufacturing system, smaller equipment is connected to each other. It has many
advantages such us smaller equipment footprint, less operators, reduction of human errors and lower risk
of contamination by foreign particles. On the other hand, it has some issues to be solved: new investment
in equipment, establishment of high-level quality monitoring systems using PAT tools, and fewer NDA or
ANDA applications in the past. Shionogi Pharma has introduced a continuous manufacturing system and will
file NDA applications for products manufactured by this system as early as possible. Shionogi Pharma’s
experiences in the continuous manufacturing will be used for customer support.
Advantages of continuous manufacturing technology
| Development stage |
Continuous manufacturing does not need scale up study at the various stages of development. It
has following advantages: |
|---|---|
| Commercial manufacturing |
Manufacturing period can be shortened because the manufacturing can be conducted
continuously. |
The picture below is CTS-MiGRA continuous manufacturing system developed by Powrex Corporation. Since Shionogi Pharma introduced this system, it can seamlessly manufacture both clinical trial drugs and commercial products, without site changes accompanied by many kinds of losses.

Shionogi Pharma started its business on April 1, 2019 on a mission of becoming a technology-developing MNOZUKURI*2 company (CDMO*3) trusted by its clients. Shionogi Pharma can provide one-stop and full-range services including development of manufacturing methods for APIs and drug formulations, and commercial manufacturing of drug products. Shionogi Pharma can also conduct analytical method development and equipment designing support using pharmaceutical engineering technologies.
*2 MONOZUJURI: “MONO” means things and “ZUKURI” means making. MONOZUKKURI is a Japanese traditional
concept of making things with enough experiences and skills.
*3 CDMO: Contract Development Manufacturing Organization
Topics
- 2022/09/09
- TopicsIntroduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
- Introduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
- 2022/09/09
- TopicsA novel freeze-drying method using controlled freeze-drying technology
- A novel freeze-drying method using controlled freeze-drying technology
- 2022/09/09
- TopicsAnti-cancer Drugs Exposure Evaluation Services
- Anti-cancer Drugs Exposure Evaluation Services
- 2022/09/09
- TopicsManufacturing facilities for highly potent APIs
- Manufacturing facilities for highly potent APIs
- 2022/09/09
- TopicsIntroduction to Technologies for Continuous Manufacturing of Drug Proucts
- Introduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
