- HOME
- Site & Plants
- Amagasaki Office
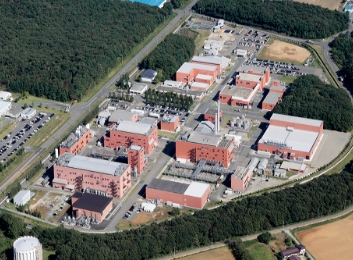
Amagasaki Office
Main Site for R&D and Investigational New Drug Manufacturing
At Amagasaki Office, we conduct pharmaceutical product development and manufacturing of investigational new drugs, as well as development of new technology, utilizing our rich experience in global new drug development.

1-3, Kuise Terajima 2-chome, Amagasaki, Hyogo 660-0813 Japan
+81-6-6401-1221
Japan
PMDA
USA
FDA
R&D
We can provide services for any of clients’ needs around pharmaceutical CMC research and development activities, such as API and process development, formulation development, package development, development of analytical methods and specifications and regulatory application support.
Investigational New Drug Development
We can provide services for any of clients’ needs around pharmaceutical CMC research and development activities, such as API and process development, formulation development, package development, development of analytical methods and specifications and regulatory application support.
API
API Manufacturing Building A
Building A is our manufacturing plant for investigational new drugs and intermediates. The plant has 2 multi-purpose manufacturing lines for API and intermediates and ISO class 7 clean room to enable trial manufacturing of a wide range of drugs.
Major Facilities
| Manufacturing Area |
Reactor with Distiller (200~500L GL·SS) |
|---|---|
| Clean Room (ISO Class7) |
Reactor Distiller (300L GL) |
※GL: Glass Lining TL: Teflon Lining SS:Stainless Steel

Building B
API Manufacturing Building B
Building B is for manufacturing of investigational new drugs and intermediates and with 3 manufacturing lines, it has a larger manufacturing capacity than Building A. The manufacturing equipment line-up includes state of the art PAT equipment such as FBRM (Focused Beam Reflectance Measurement) to enable real time process monitoring. The clean room is equipped with Jet Mill to handle clients’ requests from manufacturing to pulverization of investigational new drugs.
Major Facilities
| Manufacturing Area |
Reactor Distiller (200~1000L GL·SS) |
|---|---|
| Clean Room (ISO Class7) |
Reactor Distiller (1000 GL) |
※GL: Glass Lining TL: Teflon Lining SS:Stainless Steel
Formulation and Packaging

Formulation and Packaging/Manufacturing Building
Formulation and packaging/manufacturing building is seven stories high. The lower levels are primarily for manufacturing and experimental area for investigational new drug dosage and packaging specifications design, whereas the higher levels are primarily GMP manufacturing area for drug formulation and packaging with focus on investigational new drugs.
Major Facilities
| Aseptic Filling Lyophilization |
Vial Washer |
Vial Dry Heat Sterilizer |
|---|---|---|
| Pulverization |
Spiral Jet Mill |
Sample Mill |
| Granulation |
10L Type Agitating Granulator |
50L Type Agitating Granulator |
| Sizing |
Power Mill |
Fitz Mill |
| Mixing |
10L Total Capacity V Type Mixer |
22L Total Capacity Agitating Granulator |
| Dry Granulation |
Roller Compactor |
|
| Capsule Filling |
5 Type Capsule Filler |
Capsule Deduster |
| Compression |
12 Station Compression |
45 Station Compression |
| Spray Dry |
Spray Dryer (Organic Solvent Capable) |
|
| Coating |
48 Type Coating |
80 Type Coating |
| Packaging |
Semi-automatic PTP Packaging (Sealer) |
Semi-automatic PTP Packaging (Trimmer) |
Analysis

In addition to quality testing of investigational new drugs, we can provide various services around quality from investigational new drug API and formulation analysis methods and standards design appropriate for each development stage, stability testing for regulatory application, approval application, and to transfer of analysis technology to commercial manufacturing locations.
Major facilities
| Physiochemical Analytical Instruments |
Liquid Chromatograph (HPLC,UPLC)/Mass Spectrometer |
Particle Size Analyzer |
|---|---|---|
| Microbial Testing Instruments |
Aseptic Testing Isolator |
Genetic Analyzer |
| Surface Area Analysis Instrument |
Scanning Electron Microscope |
|
| Sample Storage |
Low Temperature Thermo-Hygrostat |
Prefabricated Thermo-Hygrostat |