- HOME
- Information
- Manufacturing facilities for highly potent APIs
Manufacturing facilities for highly potent APIs
Introduction to the manufacturing building for highly potent APIs in Tokushima Plant
Highly potent substances evoke given responses at low concentrations. These highly potent substances are
often used to manufacture drug products. The demand for facilities that can safely manufacture such
substances has recently been increasing from the viewpoint of the protection of workers, reduction of
environmental loads and prevention of cross contamination.
Tokushima Plant of Shionogi Pharma Co., Ltd. (Shionogi Pharma, hereafter) has a building which can
manufacture highly potent APIs classified in the hazard level 5 (OEL*1 0.1-10 μg/m3). This building has
successful inspection history by FDA and other foreign health authorities. It has two separated synthetic
facilities and milling facilities that can manufacture different kinds of high potent substances in
parallel. It can manufacture high potent APIs of about 1-10kg/batch for commercial use or clinical trials
in compliance with GMPs. If you want to find a facility capable of manufacturing relatively small amount
of high potent drug substances, contact us Shionogi Pharma.
*1: Occupational Exposure Limit
Overview of the double barrier system
The risk of exposure to a chemical is defined as the multiplication of the hazardousness of the chemical
by its amount of exposure. The control level of each chemical powder handling is established based on its
risk. Our double barrier system consists of a primary barrier and a secondary barrier:
Primary barrier: containment equipment (e.g. negative pressure isolator that locally contains the source
of dust generation).
Secondary barrier: negative pressure rooms with a HEPA*2 filter for exhausted air (environment surrounding
the equipment for equipment)
* 2: High Efficiency Particulate Air Filter
Shionogi Pharma’s Anti-cancer Drug Exposure Evaluation Services
1) Primary barrier (equipment for containment)
This section provides an overview of the containment equipment of the building for high potent APIs.
Operations which easily generate powder dust such as drying, handling of micronized crystals in the
milling process and weighing and subdivision are carried out in a negative-pressure isolator to achieve
high level of containment.
As in-line monitoring system (for crystallization and milling processes) by PAT*3 has been proactively
employed to handle high potent substances, high-level quality assurance has been achieved.
*3: Process Analytical Technology: A system for designing, analyzing, and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality. (ICH Q8(R2)

Globe box (isolator) for charging materials
Handling of dry powder
・Use of an isolator: maintains negative pressure inside (about -150 Pa)
・ HEPA filters for supplied and exhausted air (double filters for exhausted air)
・ Transfer of materials by bag-in bag-out system
・ Use of WIP and CIP systems
(WIP: wet in place, CIP: cleaning in place)

Soft isolator for charging materials
Handling of wet powder
・ Use of single-use techniques → Reducing the risk of cross-contamination
・ No need of cleaning validation → Reducing workers/analysts tasks
・ Good visibility and flexibility* → Good workability
(*bending or sagging characteristics)
In-line monitoring of crystallization process
The crystallizer is equipped with PAT tools (in-line particle size distribution tester and particle image analyzer) to reduce sampling operations and achieve high-level quality assurance.

PAT tool (in-line particle size distribution measurement)

Horizontal centrifugal separator
Separation of crystal slurry
・ Use of horizontal centrifugal separator for poorly filterable crystal slurry (the
machine body is placed outside the clean room)
・ Automatic cake scraping function (possible to automatically discharge cake without opening the
basket)
・ A liner tube and a high-level containment crimp are used for the exit for wet crystals

Filter dryer (isolator for crystal outlet)
Filtration and drying of crystal slurry
・ Only crystal outlet is contained by an isolator
・ Features of the filter dryer
① Filter plate has also heating function. Good heat transfer function leads to highly
efficient drying.
② Equipped with agitating blades to achieve uniform crystals
③ High yield is achieved by collecting remaining crystals by a gloved operation after automatic
discharging
Dry milling operation
・ Use of a counter-jet mill contained in an isolator
・ Capable of dry milling under a nitrogen atmosphere (anti-explosion).
・ PAT tool (in-line particle size analyzer) is used to assure high-level quality assurance.
・ WIP and cleaning with organic solvents can be conducted.
・ Isolators for milling and weighing and subdividing are connected with each other to enable a range of
operations from milling to weighing and subdividing to collecting of milled materials into drums in a
contained environment.

Counter Jet Mill (Grinding Isolator)

Weighing and subdividing isolator (inner image)
2) Secondary barrier
Local containment is conducted by the primary barrier. The secondary barrier is used for just in case
where some leakage occurs from the primary barrier for some reason. To contain the leakage, as the
secondary barrier, air pressure of all rooms relating to the manufacturing are controlled to maintain
negative pressure. The HVAC system for each room which has contained equipment inside is equipped with
HEPA filters for supplied and exhausted air. This HVAC system uses once-through air system (all fresh air
system). Particularly for rooms where dry powder is used, double HEPA filters are used for the exhausted
air. A bag-out system is used for the HEPA filters so that workers can safely change the filters.
The figure below shows as an example of the secondary barrier the layout of the changing room and pass
room leading to the room where powder is handled.
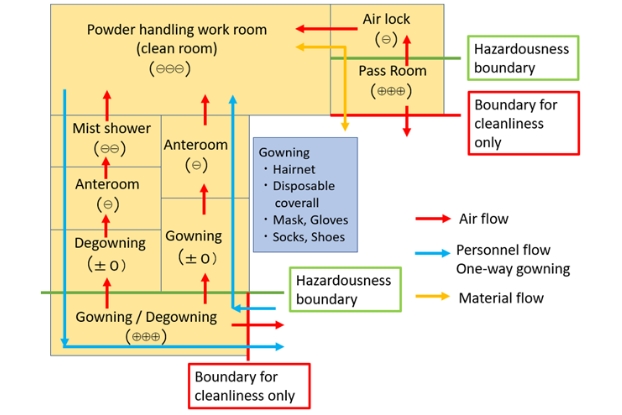
Examples of the secondary barrier
The blue lines show personnel flow. Personnel flow is separated between entering and leaving the workroom
to enable one-way changing. Workers move along these lines and change their clothing to enter and leave
the work room where powder is used. The yellow line shows material flow. The material flow is not
separated between entering and leaving. As for air flow (red lines) and room pressure, the pressure of
gowning/degowning room and that of the pass room is the highest and the air flows from these rooms into
the workroom where powder in handled.
This characteristic layout shows construction design that meets both requirements of containing high
potent APIs, our highest priority, and requirements for the secondary barrier. The green lines mean the
boundaries between hazardous area and non-hazardous area. The gowning/degowning room and the pass room are
being used with the concept of not bringing out high potent substances in mind.
Qualification of containment equipment
Qualification of the containment equipment as the primary barrier was implemented following SMEPAC*4
published by ISPE (International Society for Pharmaceutical Engineering) using placebo powder (lactose).
Measuring procedure, sampling points, and analysis were based on SMEPAC.
As for actual powder, monitoring of the scattered powder was conducted outside the secondary barrier and
the air exhaustion system from the viewpoint of prevention of cross contamination and protection of
environment.
Shionogi Pharma provides services not only for contract manufacturing but also for supporting our
customers regarding designing of facilities that handle high potent substances, consulting about risk
assessment. Shionogi Pharma also provides analytical services such as exposure assessment and scattered
dust measurement.
Don’t hesitate to contact us if you are interested in our services.
* 4: Standardized Measurement of Equipment Particulate Airborne Concentration
Primary Equipment of High Potent API Building Tokushima Plant
Primary equipment of High Potent API Building
| Equipment name | Capacity / Filtration area | Material | Remarks |
|---|---|---|---|
| Reactor | 50L | Glass lining | Glove box for charging |
| Reactor | 300L | Glass lining | Glove box for charging Capable of middle-pressure reaction |
| Distillation reactor | 50L | Glass lining | |
| Distillation reactor | 100L | Glass lining | |
| Distillation reactor | 200L | Glass lining | |
| Distillation reactor | 400L | Glass lining | |
| Distillation reactor | 150L | SUS316L | |
| Distillation reactor | 400L | MA22 (equivalent to Hastelloy® C-22) |
|
| Distillation reactor (Crystallizer) |
400L | Glass lining | Isolator PAT tools (FBRM, PVM®*5) |
| Distillation reactor (Crystallizer) |
50L | Glass lining | Isolator PAT tools (FBRM, PVM®*5) |
| Filter press | 0.2m² | SUS304 + PFA lining | Isolator |
| Centrifugal separator | 40L | MA22 (equivalent to Hastelloy® C-22) |
Horizontal type |
| Filter dryer | 150L (0.2m²) |
MA22 (equivalent to Hastelloy® C-22) |
Isolator Agitating blade |
| Milling machine | - | SUS316L | Isolator PAT tool (particle size analyzer) |
*5: FBRM (Focused Beam Reflectance Measurement), PVM ® (Particle Vision and Measurement):Mettler Toledo’s Particle Analyzers
Shionogi Pharma started its business on April 1, 2019 with the mission of becoming a
technology-developing MONOZUKURI* 6 company (CDMO* 7) trusted by customers. Shionogi Pharma can offer
full-range services including development of analytical procedures and support making use of
pharmaceutical engineering technologies to equipment designing in addition to development of manufacturing
methods of drug substances and products and their commercial manufacturing.
Tokushima Plant conducts contract manufacturing of APIs and intermediates for clinical trials and
commercial APIs and intermediates on a full range of scales from small to large scales.
Shionogi Pharma will be able to offer contract manufacturing or solution services responding to our
customers’ requests. Contact us freely.
* 6: MONOZUKURI : a combination of 'mono' meaning thing and 'zukuri' meaning the act of making. It simply means craftsmanship or manufacturing and has come to be used as a buzzword in industry and mass media to embody the Japanese spirit and history of manufacturing.
https://en.wikipedia.org/wiki/Monozukuri
* 7: CDMO: Contract Development Manufacturing Organization
Topics
- 2022/09/09
- TopicsIntroduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
- Introduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
- 2022/09/09
- TopicsA novel freeze-drying method using controlled freeze-drying technology
- A novel freeze-drying method using controlled freeze-drying technology
- 2022/09/09
- TopicsAnti-cancer Drugs Exposure Evaluation Services
- Anti-cancer Drugs Exposure Evaluation Services
- 2022/09/09
- TopicsManufacturing facilities for highly potent APIs
- Manufacturing facilities for highly potent APIs
- 2022/09/09
- TopicsIntroduction to Technologies for Continuous Manufacturing of Drug Proucts
- Introduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
