- HOME
- Information
- A novel freeze-drying method using controlled freeze-drying technology
A novel freeze-drying method
using controlled freeze-drying technology
Shionogi Pharma developed a novel freeze-drying technology (ice-fog method) that controls the freezing state to enhance product homogeneity as well as reduce drying time.
Freeze-drying technologies are normally used when pharmaceutical products are unstable in solution or in the formulation design of sterile drug products.
Freeze-drying process mainly consists of a freezing process in which solution is frozen to form ice crystals, a primary drying process in which sublimation drying is performed and a secondary drying process in which combined water is removed by evaporation. Among them, the freezing process is a critical process that affects the subsequent primary drying by sublimation and determines the final product quality.
In a conventional freezing process, solution is excessively frozen by supercooling. Formed crystals in this process are usually small if the freezing speed is not appropriate, which makes stronger the sublimation resistance in the primary drying process (meaning longer drying time). Additionally variation of the timing of freezing and the size of ice crystals are likely to occur between products placed in the chamber of the freeze-dryer, affecting the homogeneity of the products.
The ice-fog method is a technique to control freezing by introducing ice fog (made by cooling vapor with liquid nitrogen into fog) into the supercooled solution (close to the freezing point) to induce the formation of ice nuclei. Freezing timing can be controlled by using this technique, which is useful to make ice crystals more homogenous and larger 1)and shortening of drying time and product homogenization are expected (see Fig. 1 and the video below).
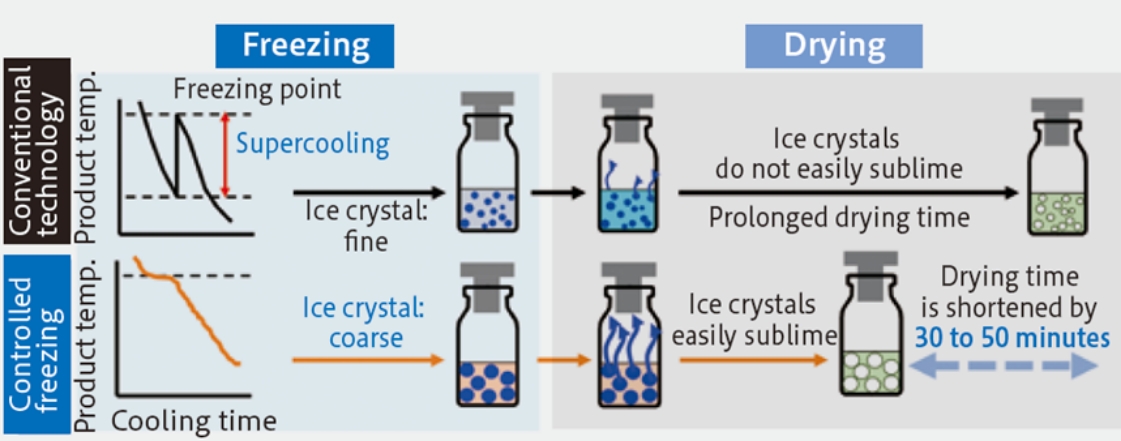
Figure 1. Effect of controlled freezing on crystal size and drying time
1) KAWASAKI Yoshinori, “Design of Lyophilization Process”, Journal of Japan Society of Pharmaceutical
Machinery and Engineering, Vol.24 No2, p39-p51, 2015
※See video for freeze drying by ice-fog method.
Shionogi Pharma is conducting research and development of freeze drying processes including ice- fog method in collaboration with Shionogi & Co., Ltd. with a view to their application to manufacturing of drugs. Shionogi will make proposals to meet customer’s requests.
Shionogi Pharma started its business on April 1, 2019 with the mission of becoming a
technology-development CDMO※2 trusted by customers. Shionogi Pharma can provide customers with FULL-RANGE
and ONE-STOP SERVICES including development of analytical procedures and designing of equipment using its
pharmaceutical engineering technologies as well as development of manufacturing methods of APIs and drug
products and their commercial production. Shionogi Pharma is providing contract and solution services.
CONTACT US
※2 CDMO: Contract Development Manufacturing Organization
Topics
- 2022/09/09
- TopicsIntroduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
- Introduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
- 2022/09/09
- TopicsA novel freeze-drying method using controlled freeze-drying technology
- A novel freeze-drying method using controlled freeze-drying technology
- 2022/09/09
- TopicsAnti-cancer Drugs Exposure Evaluation Services
- Anti-cancer Drugs Exposure Evaluation Services
- 2022/09/09
- TopicsManufacturing facilities for highly potent APIs
- Manufacturing facilities for highly potent APIs
- 2022/09/09
- TopicsIntroduction to Technologies for Continuous Manufacturing of Drug Proucts
- Introduction to High-Sensitivity Analysis Techniques for Elemental Impurities ~ Providing solutions for ICH Q3D
