- HOME
- Site & Plants
- Kanegasaki Plant
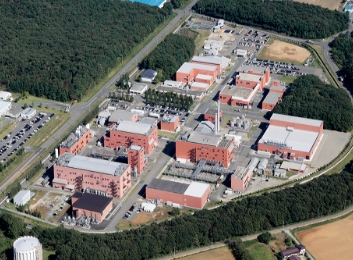
Kanegasaki Plant
Integrated manufacturing site specializing in cancer pain treatment drugs, and cephem/carbapenem antibiotics
The capacity of Kanegasaki Plant is approximately 100 tons/year for antibiotics API, 17 million vials/year for vial formulation and 420 million tablets/year for tablet formulation, where manufacturing is conducted under adequate confinement and aseptic technology to guarantee high quality to meet safety requirements and to give a sense of security to clients. Our past performance of clearing many overseas and domestic audits can assure our clients to place trust on us and enable smooth market introduction of products.

7 Nishine Moriyama, Kanegasakai City, Iwasa-gun, Iwate 029-4503, Japan
TEL +81-197-44-5121
Japan
PMDA
USA
FDA
EU
EMA
South Krea
MFDS
Saudi Arabia
SFDA
Taiwan
TFDA
Brazil
ANVISA
South Africa
MCC
Cephem Antibiotics API/Intermediates Manufacturing Building
We manufacture high quality Cephem antibiotics API and intermediates. In Compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP, the plant is capable of supplying products globally to the USA, EU and Asian countries in addition to domestic market.

Production Control System

Multiple Large Capacity Reactors
General Work Area Major Facilities
| Reactors |
300~10,000L(SUS、GL) |
|---|---|
| Low Temperature Reactors |
10,000L(GL、-80℃) |
| Separators |
Centrifuge (Low Exhaust type): 60x40inch,48×38inch(TL) |
| Rectifying Column |
φ1,200×6,000(TL) |
| Adsorbing Column |
Φ850×2,500(TL) |
| Dryer |
Conical: 5,000L(GL), 6,000L(GL) |
General Work Area Major Facilities
| Reactor Crystallizer |
Reactor: 500L (GL) |
|---|---|
| Separator |
Centrifuge (Top Exhaust type): 42inch (TL) |
| Dryer |
Conical: 300L(GL) |
| Pulverizer |
Power Mill Oscillator (SUS) |
Cephem Antibiotics Aseptic Formulation/Packaging Building
We have facilities for high quality cephem antibiotics injectable vial formulation (lyophilized formulation.) In compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP requirements, we can manufacture high quality aseptic products for the global market.

Building

Three (3) units of largest-scale Lyophilizer in Japan
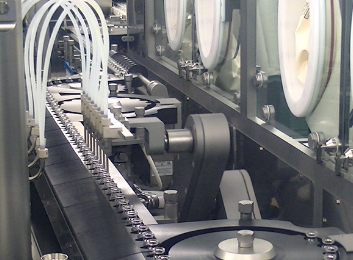
Vial filling line
Major Facilities
| Sterilization / Decontamination |
Autoclave |
|---|---|
| Solution preparation system |
Solution preparation tank (350L, SUS), Automatic pH control system, Final mixing tank (1100L, SUS) |
| Vial Filling Line |
Vial Washer, Depyrogenation tunnel, Vial filling stoppering machine (solution) |
| Lyophilizer |
Lyophilizer, Automated vial loading systems |
| Vial Capper |
RABS |
| Vial Exterior washing |
Exterior Washer |
| Inspection |
100% visual inspection |
| Vial Packaging |
Vial packaging line |
Cephem Antibiotics Solid Oral Formulation/Packaging Building
We have facilities for high quality cephem antibiotics solid oral formulation (tablets, capsules, granules.) In compliance with PIC/S GMP requirements, we can manufacture high quality products for the global market.

Tablet Press

PTP filling packaging line

PTP packaging line
Major facilities
| Mixing |
Boat-shaped vibrating screen classifier |
|---|---|
| Sifting/Classifier |
Turbo cleaner |
| Granulator |
Agitating granulator |
| Dryer |
Fluidized-bed dryer (120type) |
| Pulverization / Sizing |
Jet Mill, Power Mill, Pin Mill |
| Tableting |
Tablet Press (45 stations) |
| Capsule Filling |
Capsule filling equipment (200 type) |
| Coating |
Coating equipment (130 type) |
| Inspection |
Exterior inspection equipment (granules) |
| Printer |
Print inspection equipment (Tablet) |
| Printing Process |
Poly bottle/Glass bottle filling packaging line (fine grains) |
Carbapenem Antibiotics API/Intermediates Manufacturing Building
We have facilities for high quality carbapenem antibiotics formulation (API and intermediates.) In compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP requirements, we can manufacture high quality aseptic products for the global market.

Aseptic isolator

Vibrating dryer
General Work Area Major Facilities
| Preparation Equipment |
1,000L(SUS) |
|---|---|
| Reactor |
300~4,000L(SUS、GL、HC) |
| Reactor Rectifier |
1,500~3,000L(SUS、GL) |
| Extractor |
1,000L(SUS) |
| Separators |
Centrifuge: φ900(HC) |
| Dryer |
Conical: 1,000 (GL) |
Product Area major Facilities
| Preparation Equipment |
1,500L(SUS) |
|---|---|
| Reactor Crystallizer |
Reactor: 500L(SUS) |
| Solution Mixing Tank |
1,200L(SUS) |
| Aseptic Facilities |
Depyrogenation ultrafiltration equipment: 50m2 |
| Separators |
Centrifuge: 30inch(TL) |
| Dryer |
Vibration Fluid Dryer: 120L(SUS) |
Carbapenem Antibiotics Aseptic Formulation/Packaging Building
We have facilities for high quality carbapenem antibiotics injectables vial formulation (Aseptic powder filled formulation.) In compliance with J-GMP, c-GMP, EU-GMP, and PIC/S GMP requirements, we can manufacture high quality aseptic products for the global market.

Outside View

Vial Powder Filling facility
Major Facilities
| Sterilization/Decontamination |
Autoclave |
|---|---|
| Vial Filling Line |
Vial Washer, Depyrogenation tunnel, Accofil model Powder filling stoppering machine, Capper, Exterior Washer |
| Inspection |
100% Visual Inspection |
| Packaging |
Vial packaging line |
Cancer Pain Treatment Drugs API/Formulation (solid/injectables) / Packaging Building
We have manufacturing facilities for high quality cancer pain treatment drugs. In compliance with domestic API GMP, we can manufacture high quality products including injectable ampule formulation and solid oral formulation (tablets, powders.)