- HOME
- Site & Plants
- Itami Plant
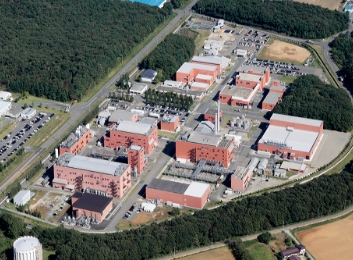
Itami Plant
Manufacturing Site for Injectables and Liquids
With the aseptic product manufacturing technology that we have accumulated for more than 80 years, the Itami Plant can provide comprehensive services, from the manufacturing of clinical trial drugs to commercial products, as a manufacturing site for high potent and non-hazardous injectables and liquids.Equipped with manufacturing facilities for injectables using drug delivery system (DDS) technology, it can develop and manufacture high-value-added drug products through the fusion of aseptic product manufacturing technology and DDS-based formulation manufacturing technology.With technical capabilities that we have gained over many years, we provide our customers with a sense of security and smooth launch.

4-323 Senzo, Itami City, Hyogo 664-0898, Japan
TEL +81-72-778-7501
Japan
PMDA
High Potent Injectables Manufacturing Building
Formulation

Itami Plant High Potent Injectables Manufacturing Building
With two manufacturing lines for high potent injectables (manufacturing line for vial injectables / manufacturing line for vial lyophilized injectables) that can handle high potent injectables with a hazardous level of up to 5 (OEL 0.15 μg/m³), we can provide injectables in glass vials as well as plastic vials.
In addition, advanced cross-contamination prevention with the latest single-use system and advanced nitrogen replacement in the vial headspace with a vacuum capper are possible.
The High Potent Injectables Manufacturing Building meets the requirements of three-region (Japan, USA, and EU) GMPs and PIC/S GMP and is capable of advanced product manufacturing.
Major Facilities
| Weighing |
Negative pressure isolator for weighing |
|---|---|
| Solution Mixing |
Solution mixing tank (100 L, single use) |
| Filling |
<Manufacturing line for vial injectables> |
| Lyophilization |
<Manufacturing line for vial lyophilized injectables> |
| Capper |
<Manufacturing line for vial injectables> |
| Washing |
Exterior washer |

Manufacturing line for vial injectables

Manufacturing line for vial lyophilized injectables
Inspection / Packaging

Shrink / tack label packaging combined machine
For packaging, the use of shrink labels and of protectors at the vial bottom makes it possible to prevent vials from breaking and prevent drug solution from scattering.
It is also possible to meet the need for drug exposure prevention in medical settings by adopting a packaging design with a cushioning function for preventing vial breakage.
Major Facilities
| Inspection |
100% visual inspection |
|---|---|
| Packaging |
Shrink / tack label packaging combined machine |
High Potent DDS-based Injectables
Since we started the contract manufacturing of high potent DDS-based injectables in 2004, we have accumulated a track record of manufacturing more than 15 items.
The High Potent Injectables Manufacturing Building has a manufacturing area dedicated to DDS-based injectables, where multiple pieces of equipment (GMP-compliant equipment) for manufacturing DDS-based injectables have been introduced. We are capable of manufacturing a wide range of products, from clinical trial drugs to commercial products.
Major Facilities
| Particle Sizer |
Extruder, Microfluidizer, Microfluidic device |
|---|---|
| Ultrafiltration Equipment |
Available for various ultrafiltration membranes |
Non-Hazardous Injectables / Liquids Manufacturing Building
Formulation

Itami Plant Non-Hazardous Injectables / Liquids Manufacturing Building
This is a manufacturing building for injectables and liquids for non-hazardous drugs.
Equipped with an integrated manufacturing line for vials and ampoules for injectables and a manufacturing line for bottled solutions (solutions for inner and external use) for liquids, it is capable of manufacturing highly viscous liquids, such as syrups, and liquids with weak antiseptic properties.
It also has an integrated manufacturing line for filling and packaging jelly formulations and is capable of manufacturing jelly formulations that meet the sterility test requirements.
Major Facilities (Injectables)
| Solution Mixing |
<Manufacturing line for vial injectables> |
|---|---|
| Filling |
<Manufacturing line for vial injectables> |
| Capper |
Vial capper |
Major Facilities (Liquids)
| Solution Mixing |
<Manufacturing line for bottled solutions> |
|---|---|
| Filling |
<Manufacturing line for bottled solutions>
|

Manufacturing line for vial injectables

Manufacturing line for ampule injectables

Manufacturing line for bottled solutions

Manufacturing line for jelly formulations
Inspection / Packaging
Injectables can be inspected by automated inspection equipment and mechanically packaged with a labeler and a cartoner.
All products that cannot be inspected by automated inspection equipment are visually inspected by visual inspectors.
All liquids are visually inspected by visual inspectors.
Jelly formulations are processed by serial processing equipment that performs filling and inspection, as well as packaging at a speed of up to 200 bottles per minute.